Editor’s note: This article was written prior to the European Parliament vote on 17 December 2025, which approved a 12-month delay to the implementation of the EU Deforestation Regulation (EUDR). The content is currently under review to reflect this change.
As part of our ongoing EUDR Lunchtime Series, our Head of Market Development, Anna Roberts, hosted a dedicated session on transition periods and stocks under the EU Deforestation Regulation (EUDR).
The session attracted many thoughtful and practical questions from participants. We’ve compiled them below, alongside our responses, so others preparing for compliance can benefit from the discussion.
⚠️ Please note: these answers reflect our current understanding as of September 25th 2025. Guidance and enforcement practices continue to evolve, and we recommend monitoring updates from the European Commission and industry associations.
Helpful resources:
- August 2025 Guidance Document
- Official Regulatory Text
- Official EUDR FAQs
- List of EUTR commodities from Preferred By Nature
Questions relating to product transformation
1. If I am an SME and I am buying material with HS code 4809 and I make available on the EU market my product which is is 4811. Do I need to create a new DDS even though the 2 first digits are the same (48XX) or can I forward the reference numbers I have received from my supplier for the 4809 material? The question is because then the DDS would be listing a product under 4809 but I (an SME) is making available a product under 4811. Or is this not an issue.
SME downstream operators are not required to exercise due diligence for relevant products contained in or made from relevant products that have already been subject to due diligence and for which a Due Diligence Statement (DDS) has already been submitted.
If your product (HS 4811) is made entirely from material (HS 4809) that was already subject to due diligence by your supplier (i.e., all product inputs are already covered by upstream DDS), you are exempted from exercising due diligence and submitting a new DDS.
Please see the table from the EC’s guidance document below.
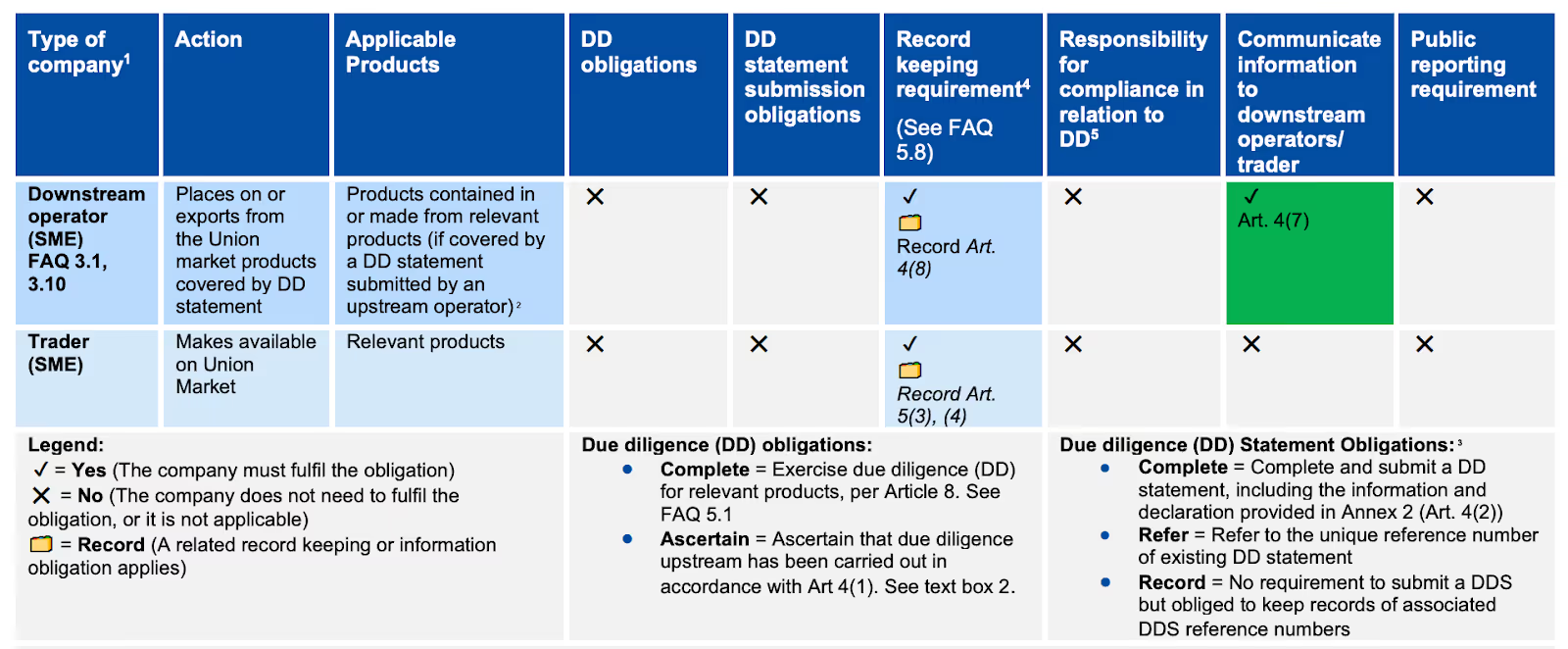
If however you purchase 4809 from non-EU suppliers and there are no upstream DDS, you would be obligated to conduct full due diligence and submit DDS for the inbound products. You can then reference the inbound DDS when you sell to your EU customers.
Questions relating to wood-based products and EUTR
2. So for timber, EUDR only applies when it is either:
- 1) Harvested before 29 june 2023 and brought on the market from 31 december 2028 - correct
- 2) OR - harvested between 29 june 2023 and 30 december 2025 and brought on the market from 30 december 2025 - correct, if the goods are not placed on the EU market during the transition period (29 June 2023 - 30 December 2025)
- 3) OR - harvested after 30 december 2025 - correct.
So my question is: How to identify this to the customer? Do we seperate the products from each other? Do some products get a TRACES code as they are in scope of the EUDR, and others not (EUTR)? So in the end, does the customer get an invoice with products where for example 30 pieces are in scope of the EUDR and 50 in scope of EUTR?
When you place a mixed batch on the market, a DDS is only required for the portion of the stock that is subject to EUDR. The EUTR products do not need a DDS. In the case of the first scenario, you can use the exemption code Y132 on customer documents.
While the regulations do not explicitly require the physical separation of EUTR and EUDR stock, you must be able to prove which items fall under which category. Therefore, you must keep records, which essentially necessitates a reliable traceability system.
Your customer may ask you for evidence that the goods were placed on the market before or during the transition period. This can take the form of bills of lading, customs declarations, dispatch notes, etc.
As only the products subject to EUDR will have a DDS and an associated reference number, your invoices or delivery notes will need to distinguish between the two types of stock by indicating which part of the order is covered by the EUTR and which is covered by the EUDR, along with the corresponding DDS reference numbers. This could be done on Sales Orders, dispatch notes, packing lists, etc. The important thing is to make sure it’s clear which products are covered by EUDR and which are not.
Some of our customers are approaching this in a similar way to when they detail the certification status of products (e.g., 70% PEFC; FSC Mix) on item descriptions.
3. For medium and large sized companies. If we ship paper that was made from Korean pulp, arrives in France on the 12th of December 2025 and is placed on the market on the 12th of December. Do they need to comply with the EUDR if the goods are sold to their clients in January 2026? Or is the EUTR ok?
No, the product does not need to comply with the EUDR. It was placed on the EU market on 12th December 2025, before the EUDR's application date (31 December 2025). It does need to comply with EUTR however.
When the product is subsequently sold to clients in January 2026 (which counts as an act of ‘making available’), the downstream customer’s obligation is limited to gathering and holding proof that the product was placed on the market before 30 December 2025. A customs declaration would serve as proof, according to Question 9.2 in the official FAQ.
4. If I understand correctly, for a finished good concerned by the EUTR and imported between 2023 and 2025, we need to have a EUDR DDS if we sell the product in 2026.
No. You do not need to submit an EUDR DDS for that product - a product already imported into the EU (‘placed on the market’) before 30 December 2025 falls into the transitional period. It needs to comply with EUTR but not EUDR.
When you sell the product in 2026, you are ‘making it available on the market’. For products that were placed on the market during the transitional period, FAQ 9.1 explains that the obligation for downstream actors in this scenario is limited to, “...gathering adequately conclusive and verifiable evidence to prove that the relevant commodity… used to produce such relevant product… was placed on the EU market before the entry into application of the Regulation.”
5. Just to be clear about EUTR products, harvested after 29 June 2025, imported in the EU before 30 dec. 2025: do you need a DDS 1) always at sales after 30 dec. 2025 or 2) only when products are processed after 30 dec. 2025?
The critical events here are:
- when the product was harvested; and
- when the product is ‘placed on the market’, which for an import is the date it is released for free circulation in the EU.
If the product was harvested after 29 June 2025, EUTR applies if the product is placed on the market before the EUDR application date of 30 December 2025, which is the case in this scenario.
EUDR would only apply (and you would need a DDS) if the product was placed on the market after 30 December 2025. This is covered on page 8 in the Commission’s official Guidance, and relates to timber and derived products covered by the Annex of EUTR, and Article 37(3) of the Regulation:
- For timber and timber products produced from 29 June 2023 until 30 December 2025 and:
- placed on the market before 30 December 2025, such products and their derived products must comply with the rules of the EUTR;
Therefore, your only obligation relating to EUDR is to be able to provide verifiable proof, such as a customs declaration, that it was placed on the market before 30 December 2025.
Questions relating to the obligations of non-EU businesses
6. We are a book publisher in the UK, and we sell books directly to EU consumers through our website and Kickstarter campaigns, fulfilled from a warehouse in the UK (we don't have an EU warehouse). Can we continue selling stock printed during the transition period, as the products are for personal use?
You can continue selling stock to EU customers until December 2025 without having to comply with EUDR.
After the transition period (ending 30 December 2025), you can continue to sell your pre-existing stock, but there are four key considerations:
- The exemption for 'personal use' does not apply to you as a commercial seller. It applies to your customers who may be purchasing the books for personal / private consumption.
- If your stock comes from in-scope raw materials which were already placed on the EU market (i.e., all raw material inputs from from EU forests, pulp mills or paper mills), you do not need to comply with EUDR and will be able to use either:
- 2a. Exemption code Y132 (for stock which was placed on the EU market prior to June 30 2023)
- 2b. A ‘conventional DDS code’ which will be issued by the European Commission (and about which we are eagerly anticipating an update) for stock placed on the EU market during the transition period
- If your stock comes from in-scope raw materials which were not already placed on the EU market (e.g., not from EU forests, pulp mills or paper mills), you will be able to continue selling them to your EU customers if you provide them with the details they will need to perform their own due diligence on the products and assess them to be deforestation and degradation free (this includes geolocations). Without this level of information your customers are unlikely to be able to comply with EUDR and may be unlikely to purchase the stock.
For scenarios 2a and 2b, your customers will have to prove that they confirmed that the products were placed on the market before or during the transition period. Provision of “adequately conclusive and verifiable evidence” could include customs declarations, bills of lading, etc. (refer to FAQ 9.2).
7. We are a publisher and have UK export copies of books in the EU market already. We assumed those products would be excluded as that product is already in the EU. But we are now told (by FEP) that despite this, any new imports count as 'first placed' even though it is the same book / batch. This makes no sense, if the book is already in Germany & Spain say, but not yet Italy, why would it be 'first placed' when it is already in the EU? This is a real problem re: stock in our UK warehouse.
The advice you have received is correct. The concept of 'placing on the market' under the EUDR applies to each individual physical product, not to a product line or batch, and is defined by Art. 2(16) as “first making available of a relevant commodity or relevant product on the Union market.”
The Commission’s Guidance Document clarifies this in 1.3(a) by stating: “The concept of ‘placing on the market’ refers to each individual relevant commodity or product, not to a type of product, irrespective of whether it was manufactured as an individual unit or a series.”
This means that while some copies of your book are already on the EU market (in Germany and Spain), any additional copies still held in your UK warehouse have not yet been placed on the EU market. When you import a book from that UK stock into Italy, that specific book is being placed on the Union market for the first time. Therefore, that import event triggers the obligations under the EUDR for that individual book.
One area that we will follow up to clarify directly with you is whether the books ‘in the EU market already’ are in a warehouse and are yet to be placed on the market (i.e., not commercial transactions involving them yet), or if they are with customers. If they are in a warehouse but are yet to be sold in the EU (‘first placed on the market’), then EUDR would apply if they are sold from 31 December 2025.
8. We are a consumer goods company in the UK, if we have sold an EUDR applicable item into Europe in 2024, would we be able to prove “placing on the market” in 2026 through the customs declarations?
Yes, a customs declaration is considered valid proof that a product was placed on the EU market.
The EUDR's main obligations will apply from 30 December 2025. Any product placed on the market before this date falls within the transitional period and is exempt from the full due diligence requirements. A sale in 2024 is within this period.
Under Question 9.2 of the official FAQ on EUDR implementation, it states that, "In case of imported products, the customs declaration of the relevant commodities or relevant products in question will be accepted as evidence of having been placed on the EU market before the date of application."
Therefore, your customs declarations from 2024 will be accepted as proof in 2026 that the items were placed on the market during the transitional period.
9. We import finished chocolate products (HS1806) from suppliers based in the European Union into Great Britain. These products are covered by a DDS reference number, submitted by the EU-based exporter in compliance with the EUDR. Could you please confirm whether, under the EUDR, we are considered a Trader in this situation, given that the products have already been placed on the EU market by the EU-based exporter (i.e., the Operator)?
No, in this specific transaction because you are a UK importer, (i.e., not in the EU), you are not considered a trader or operator under EUDR, therefore you have no obligations under the Regulation.
The exception is if you are based in Northern Ireland, which per the present terms of the Windsor Framework, should be considered to be in the EU with regards to EUDR. In which case you would be a downstream trader.
If you export the products back to EU customers (who ‘re-import’ the products), then it will be beneficial commercially to you to provide the upstream DDS reference and verification numbers from your suppliers.
10. We import finished chocolate products (HS1806) from outside the EU into GB. The final product itself is not covered under the EUDR, as it is not being placed on the EU market. This chocolate could be further distributed to NI - are we required to submit a DDS for the finished product (HS1806) if it is later placed on the EU market?
Per the present Windsor Framework, Northern Ireland should be considered to be in the EU for the purposes of EUDR.
The HS code 1806 is directly in scope of EUDR. If the product is distributed to Northern Ireland for commercial purposes then the organisation placing it on the EU market will be required to submit a DDS and exercise full due diligence since the product (and raw material inputs) have not been placed on the EU market previously.
The company placing the finished product on the market will need to evidence that it has assessed the full supply chain complexity, conducted due diligence back to source (including submitting geolocations) and, if the place of harvest is deemed to be standard risk, conduct risk assessment and mitigation.
In this scenario, it will be important to determine if your business would be classed as the first operator, or if your customer would be. We will follow up to help determine this with you.
Questions relating to company size
11. We are an SME but our clients are not. Do we have to do anything specific?
This depends on two critical factors:
- Are you the first company placing the products on the EU market (upstream operator) or are you a downstream, purchasing from EU suppliers?
- Are you a trader or an operator?
There is no minimum threshold for EUDR based on company size. Yes, as an SME you have specific obligations, but these are simplified compared to a non-SME. The key ‘specific’ action is to pass on the required compliance information, including DDS reference numbers, to your non-SME clients.
However, your exact obligations depend on your role in the supply chain, as illustrated in the below table from the EC’s guidance document.
If you are an SME upstream operator or trader (you are the first to place the product on the EU market, for example as an importer):
- You have the full due diligence obligations of any operator, regardless of your size. You must therefore exercise due diligence and submit a DDS to the TRACES NT system. You must then provide the DDS reference number to your non-SME client.
If you are an SME downstream operator (you process a product already covered by a DDS into a new EUDR-relevant product):
- You are exempt from exercising due diligence and submitting a new DDS. Your key obligation is to pass the DDS reference number from your supplier to your non-SME client to allow them to fulfill their own obligations.
If you are an SME downstream trader (you buy and sell a product that has already been placed on the market):
- You are exempt from exercising due diligence and submitting a DDS. Your obligations are to keep records of your suppliers and the DDS reference numbers of the products you handle.
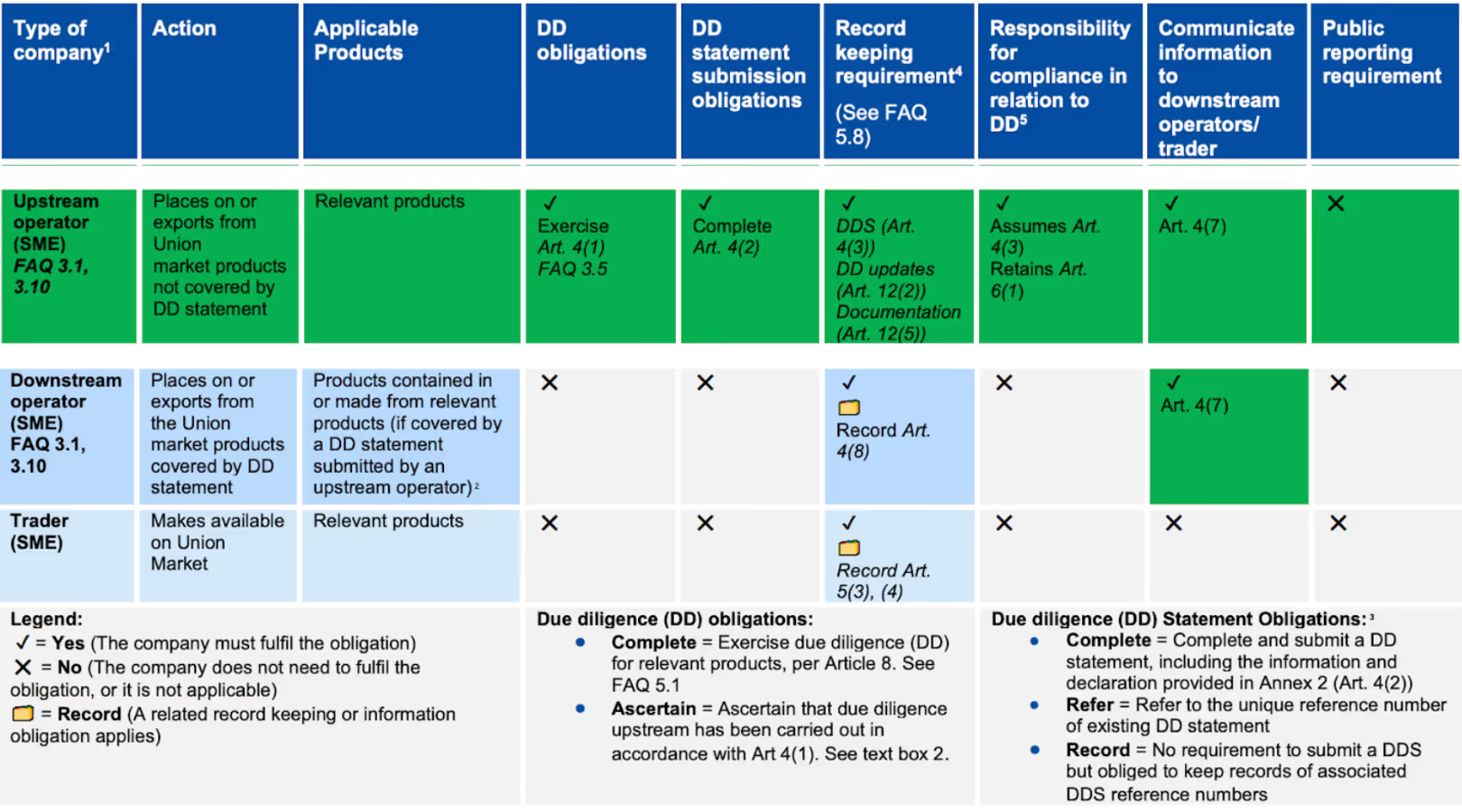
If you are a downstream SME trader, your clients cannot compel you to provide them with upstream information (such as your suppliers’ DDS reference and verification numbers), but it may be in your commercial interest to do so to maintain good relationships with your suppliers.
Referring to FAQ 3.4: Direct or indirect supply by SMEs SME traders and downstream SME operators are not obliged to collect information related to the due diligence exercise and hence are not under a legal obligation to communicate to their clients any information beyond the reference number and verification number pursuant to Art. 4(7) EUDR. This, in consequence, limits the available information that must be collected, analysed and communicated by non-SME operators and non-SME traders which are supplied directly or indirectly by SMEs. The measures taken by operators and traders when communicating information and ascertaining that due diligence was exercised should be taken into account by the competent authorities in their risk analyses.
Questions about other topics
12. For the samples from outside the EU with non commercial value. What's the process to be followed?
Under a proposed new rule, put forward by the Commission in a draft Delegated Act, samples may be exempt from the EUDR’s requirements under certain conditions. If passed, Question 2.14 of the official FAQ states that this would apply to samples of products which are:
“...of negligible value and quantity and can be consumed or used only to solicit orders for goods of the type they represent under the condition that the manner of presentation and quantity, for products of the same type or quality, rule out its consumption or use for any purpose other than that of seeking orders, are not in scope of the Regulation.
However, it is vital to note that this is a proposed change. Currently, under Article 2(18)(a) in the text of the Regulation, supplying a product free of charge (e.g. a sample) is still considered “placing on the market in the course of a commercial activity,” and would therefore be subject to due diligence requirements.
We will inform our customers as soon as there is clarification about this Delegated Act. A similar one is proposed for brochures.
Conclusion
The EUDR’s transition periods raise complex questions, particularly around stock already in circulation and the interplay between EUTR and EUDR obligations. While SMEs have simplified obligations, all actors need to understand their role in the supply chain and prepare systems to track and document compliance.
At Interu, we’re helping businesses build the traceability and reporting infrastructure they need to stay compliant, reduce risks, and maintain market access. Get in touch with us to explore how we can support your journey towards EUDR compliance.
Disclaimer: We provide a portal for the transfer of data from third party sources to individual users. You are responsible for ensuring that your use of the portal, including the data, is sufficient or appropriate for any particular use or circumstances, including taking independent professional advice as necessary. For the avoidance of doubt, you should always seek independent professional advice to confirm your compliance with applicable law.




